Biomolecules
Biomolecules
What are Biomolecules?
Biomolecules are the most essential organic molecules, which are involved in the maintenance and metabolic processes of living organisms. These non-living molecules are the actual foot-soldiers of the battle of sustenance of life. They range from small molecules such as primary and secondary metabolites and hormones to large macromolecules like proteins, nucleic acids, carbohydrates, lipids etc.
Types of Biomolecules
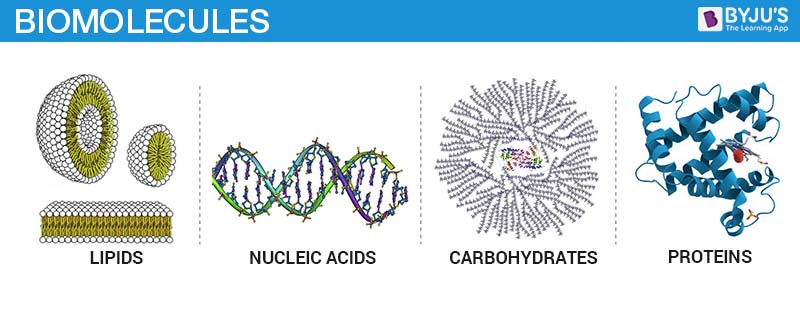
There are four major classes of Biomolecules – Carbohydrates, Proteins, Nucleic acids and Lipids. Each of them is discussed below.
Carbohydrates
Carbohydrates are chemically defined as polyhydroxy aldehydes or ketones or compounds which produce them on hydrolysis. In layman’s terms, we acknowledge carbohydrates as sugars or substances that taste sweet. They are collectively called as saccharides (Greek: sakcharon = sugar). Depending on the number of constituting sugar units obtained upon hydrolysis, they are classified as monosaccharides (1 unit), oligosaccharides (2-10 units) and polysaccharides (more than 10 units). They have multiple functions’ viz. they’re the most abundant dietary source of energy; they are structurally very important for many living organisms as they form a major structural component, e.g. cellulose is an important structural fibre for plants.
Proteins
Proteins are another class of indispensable biomolecules, which make up around 50per cent of the cellular dry weight. Proteins are polymers of amino acids arranged in the form of polypeptide chains. The structure of proteins is classified as primary, secondary, tertiary and quaternary in some cases. These structures are based on the level of complexity of the folding of a polypeptide chain. Proteins play both structural and dynamic roles. Myosin is the protein that allows movement by contraction of muscles. Most enzymes are proteinaceous in nature.
Nucleic Acids
Nucleic acids refer to the genetic material found in the cell that carries all the hereditary information from parents to progeny. There are two types of nucleic acids namely, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The main function of nucleic acid is the transfer of genetic information and synthesis of proteins by processes known as translation and transcription. The monomeric unit of nucleic acids is known as nucleotide and is composed of a nitrogenous base, pentose sugar, and phosphate. The nucleotides are linked by a 3’ and 5’ phosphodiester bond. The nitrogen base attached to the pentose sugar makes the nucleotide distinct. There are 4 major nitrogenous bases found in DNA: adenine, guanine, cytosine, and thymine. In RNA, thymine is replaced by uracil. The DNA structure is described as a double-helix or double-helical structure which is formed by hydrogen bonding between the bases of two antiparallel polynucleotide chains. Overall, the DNA structure looks similar to a twisted ladder.
Lipids
Lipids are organic substances that are insoluble in water, soluble in organic solvents, are related to fatty acids and are utilized by the living cell. They include fats, waxes, sterols, fat-soluble vitamins, mono-, di- or triglycerides, phospholipids, etc. Unlike carbohydrates, proteins, and nucleic acids, lipids are not polymeric molecules. Lipids play a great role in the cellular structure and are the chief source of energy.
What are the properties of Biomolecules?
Inorganic Compounds
An inorganic compound is a substance that does not contain both carbon and hydrogen. A great many inorganic compounds do contain hydrogen atoms.
The following section examines the four groups of inorganic compounds essential to life: water, salts, acids, and bases.
Water
As much as 70 percent of an adult’s body weight is water. This water is contained both within the cells and between the cells that make up tissues and organs. Its several roles make water indispensable to human functioning.
Water as a Lubricant and Cushion
Water is a major component of many of the body’s lubricating fluids. Just as oil lubricates the hinge on a door, water in synovial fluid lubricates the actions of body joints, and water in pleural fluid helps the lungs expand and recoil with breathing. Watery fluids help keep food flowing through the digestive tract, and ensure that the movement of adjacent abdominal organs is friction free.
Water also protects cells and organs from physical trauma, cushioning the brain within the skull, for example, and protecting the delicate nerve tissue of the eyes. Water cushions a developing fetus in the mother’s womb as well.
Water as a Heat Sink
A heat sink is a substance or object that absorbs and dissipates heat but does not experience a corresponding increase in temperature. In the body, water absorbs the heat generated by chemical reactions without greatly increasing in temperature.
Salts
Recall that salts are formed when ions form ionic bonds. In these reactions, one atom gives up one or more electrons, and thus becomes positively charged, whereas the other accepts one or more electrons and becomes negatively charged. You can now define a salt as a substance that, when dissolved in water, dissociates into ions other than H+ or OH–.
typical salt, NaCl, dissociates completely in water .The positive and negative regions on the water molecule (the hydrogen and oxygen ends respectively) attract the negative chloride and positive sodium ions, pulling them away from each other.
Many other salts are important in the body. For example, bile salts produced by the liver help break apart dietary fats, and calcium phosphate salts form the mineral portion of teeth and bones.
Acids and Bases
Acids and bases, like salts, dissociate in water into electrolytes. Acids and bases can very much change the properties of the solutions in which they are dissolved.
Acids
An acid is a substance that releases hydrogen ions (H+) in solution. Because an atom of hydrogen has just one proton and one electron, a positively charged hydrogen ion is simply a proton. This solitary proton is highly likely to participate in chemical reactions. Strong acids are compounds that release all of their H+ in solution; that is, they ionize completely. Hydrochloric acid (HCl), which is released from cells in the lining of the stomach, is a strong acid because it releases all of its H+ in the stomach’s watery environment. This strong acid aids in digestion and kills ingested microbes. Weak acids do not ionize completely; that is, some of their hydrogen ions remain bonded within a compound in solution. An example of a weak acid is vinegar, or acetic acid; it is called acetate after it gives up a proton.
Bases
A base is a substance that releases hydroxyl ions (OH–) in solution, or one that accepts H+ already present in solution. The hydroxyl ions (also known as hydroxide ions) or other basic substances combine with H+ present to form a water molecule, thereby removing H+ and reducing the solution’s acidity. Strong bases release most or all of their hydroxyl ions; weak bases release only some hydroxyl ions or absorb only a few H+. Food mixed with hydrochloric acid from the stomach would burn the small intestine (the next portion of the digestive tract after the stomach), if it were not for the release of bicarbonate (HCO3–), a weak base that attracts H+. Bicarbonate accepts some of the H+ protons, thereby reducing the acidity of the solution.
The Concept of pH
The relative acidity or alkalinity of a solution can be indicated by its pH. A solution’s pH is the negative, base-10 logarithm of the hydrogen ion (H+) concentration of the solution. As an example, a pH 4 solution has an H+ concentration that is ten times greater than that of a pH 5 solution. That is, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5. The concept of pH will begin to make more sense when you study the pH scale, as shown in figure. The scale consists of a series of increments ranging from 0 to 14. A solution with a pH of 7 is considered neutral—neither acidic nor basic. Pure water has a pH of 7. The lower the number below 7, the more acidic the solution, or the greater the concentration of H+. The concentration of hydrogen ions at each pH value is 10 times different than the next pH. For instance, a pH value of 4 corresponds to a proton concentration of 10–4 M, or 0.0001M, while a pH value of 5 corresponds to a proton concentration of 10–5 M, or 0.00001M. The higher the number above 7, the more basic (alkaline) the solution, or the lower the concentration of H+. Human urine, for example, is ten times more acidic than pure water, and HCl is 10,000,000 times more acidic than water.
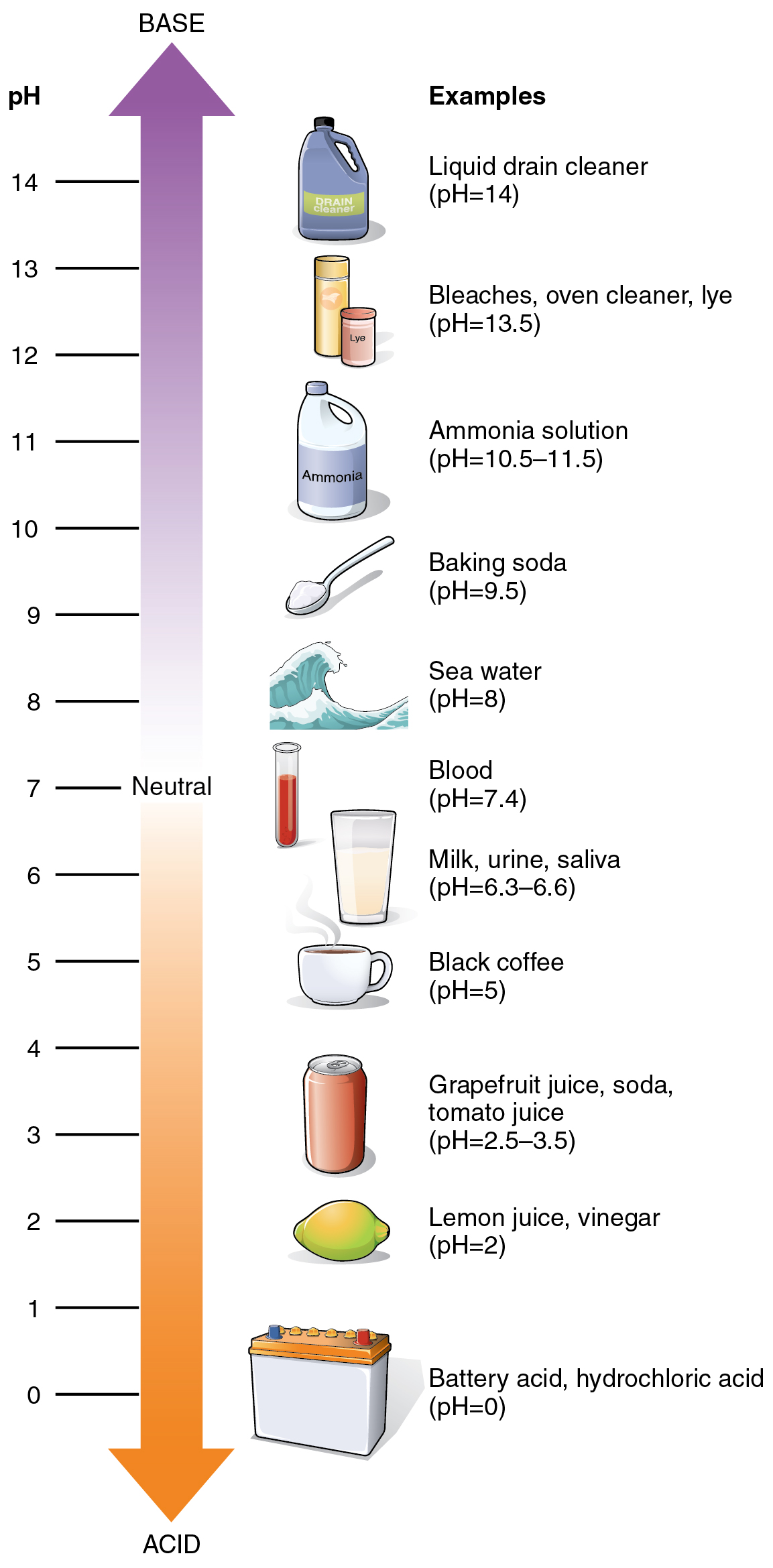
Mineral Ions
The inorganic ions of the cell, including sodium (Na+), potassium (K+), magnesium (Mg2+), calcium (Ca2+), phosphate (HPO42-), chloride (Cl-), and bicarbonate (HCO3-), constitute 1% or less of the cell mass. These ions are involved in a number of aspects of cell metabolism, and thus play critical roles in cell function.
https://byjus.com/biology/biomolecules/
https://www.slideshare.net/prof_aarif/biomolecules-15689020
.
.



Comments
Post a Comment