ESTIMATION OF HEMOGLOBIN BY CYANMETHEMOGLOBIN (CMG) METHOD
Determination of Hemoglobin by Cyanmethemoglobin metnod
you can also watch the video of ESTIMATION OF HEMOGLOBIN BY CYANMETHEMOGLOBIN METHOD
Hemoglobin is a conjugated protein present inside the erythrocytes. Hemoglobin consist of a prosthetic group named heam, which is combined with protein called globin (Hemoglobin = Heam + Globin). Other heam containing proteins in the body are Myoglobin, Cytochrome-C etc. Heam carries oxygen (O2) from the lungs to the tissue cells and carbon dioxide (CO2), the gaseous waste product from the cells to the lungs. After the normal life span of RBC (over 120 days), the red cells are destroyed by the reticuloendothelial cells (specially in the spleen) and the components of the hemoglobin undergo metabolic degeneration.
Several methods are available for the estimation of hemoglobin in the blood. These are:
- Acid heamatin method (Sahli’s method)
- Cyanmethemoglobin method.
- Alkaliheamatin method.
- Haldane’s Carboxyhemoglobin method.
- Oxyhemoglobin method.
Former two methods are commonly used for determination of hemoglobin concentration.
Clinical Significance :
A decreased in hemoglobin concentration below normal range in an indication of anaemia. An increased in hemoglobin concentration occurs in haemo-concentration due to loss of body fluid in sever diarrhea and vomiting. High value also observed in congenital heart disease (due to reduce O2 supply) in emphysema and also in Polycythemia.
Hemoglobin concentration drops during pregnancy due to haemodilution (a proportionately greater increase in plasma volume as compared to the increase in the red cell mass). Several abnormal forms of hemoglobin (variants) have been identified in the human body but only a few are common and clinically significant.
Principle :
When anticoagulated blood is mixed with Drabkin’s reagent containing potassium cyanide and potassium fericyanide. Hemoglobin reacts with potassium fericyanide to form methemoglobin which is converted to stable Cyanmethemoglobin by the potassium cyanide. The intensity of the colour is directly proportional to the concentration of the concentration of hemoglobin present in the specimen. The intensity of the colour is measured in the colorimeter at 540 nm (green filter).
Specimen :
Free flowing capillary blood or anticoagulated venous blood (EDTA or Double oxalate). The specimen need not be fasting sample.
Requirements :
- Drabkin’s reagent :
- Potassium cyanide : 100 mg
- Potassium fericyanide : 400 mg
- Potassium dihydrogen phosphate : 280 mg
- Nonidet : 1.0 ml
- Distilled water : 1000 ml
- Cyanmethemoglobin standard – 15 gm/dl
- Micropipette with micro-tips.
- Test tube.
- 5 ml pipette.
Normal values :
- Male : 13 – 18 gm/dl
- Female : 12 – 16.5 gm/dl
- Children (up to 1 year) : 11 – 13 gm/dl
- Children (10-12 years) : 11.5 – 14.5 gm/dl
- Infants (New born) : 13.5 – 19.5 gm/dl
Procedure :
- Pipettes in the tubes labeled as following :
| Reagent | Test (T) | Blank (B) |
| Drabkin’s reagent, ml
Blood, ml (well mixed) |
5.0
0.02 |
5.0
– |
- Mixed the contains in the tube labeled as ‘Test’ thoroughly and wait for 5 minutes.
- Read the absorbance of the ‘Test’ by setting ‘Blank’ to 100% T at 540 nm (green filter) in the colorimeter.
- Read the absorbance of the standard (15 gm/dl) by pipettes it directly in a cuvette.
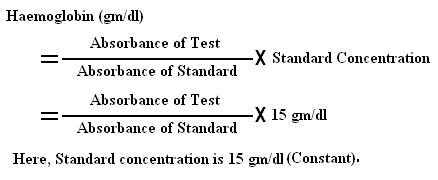
- Calculation :
Preparation of Standard Graph :
A standard graph for determination of hemoglobin can be prepared as follows :
Requirements :
- Drabkin’s reagent
- Cyanmethemoglobin standard – 15 gm/dl.
Procedure :
- Take 5 ml of Drabkin’s solution in a test tube and add 20 μl of blood. This way, we will get the dilution of 1:25. Now mix the mixture and allow to stand for at least 5 minutes. This time is adequate for the transformation of hemoglobin to hemiglobincyanide.
- Pour the test sample into a cuvette and read the absorbance of the test sample in a spectrophotometer at 540 nanometers or in a photoelectric colorimeter using a yellow-green filter. Also, read the absorbance of the standard solution. Absorbance must be read against Drabkin’s solution.
- From the formula given below, the hemoglobin value is derived.
- Pipettes in the tubes labeled as following :
| Reagent | Std. 5 | Std. 10 | Std. 15 | Blank |
| Drabkin’s reagent, ml | 3.34 | 1.67 | 0.00 | 5.0 |
| Hemoglobin Standard, ml | 1.66 | 3.33 | 5.0 | 0.00 |
- Mixed well and wait for 3 minutes and read the absorbance of colour of these Standards by setting Blank to 100% T at 540 nm (green filter) in the colorimeter.
- Prepared a graph by plotting Absorbance of standard on ‘Y’ axis and Concentration of hemoglobin Standard that is 5.0 gm/dl, 10.0 gm/dl and 15.0 gm/dl on ‘X’ axis.
- A standard line passing through origin indicates agreement with “Beer’s law”.
- This Graph can be used as a Standard graph for hemoglobin concentration determination.
Hemoglobin in gm/dl =
[Absorbance of test sample ÷ Absorbance of standard] x concentration of standard
Preparation of table and graph
The result can be obtained quickly if the table of a graph is prepared which corresponds to absorbance with hemoglobin concentration. This is markedly acceptable when a huge number of samples are daily processed on the same instrument.
For the preparation of a calibration graph, adulterate cyanmethemoglobin standards are commercially available. As another option, a standard cyanmethemoglobin solution is diluted serially with Drabkin’s solution. The concentration of hemoglobin (horizontal axis) in each dilution is arranged against the absorbance (vertical axis) on a linear graph paper. A straight line connecting the points and passing through the origin is obtained. A table can be prepared relating absorbance to the concentration of hemoglobin from the help of this graph.
Additional Information :
- Methemoglobin, Carboxyhemoglobin and sulfhemoglobin are not converted to acid haematin by reacts with 0.1(N)HCl acid.
- This method is useful for places where photometers are not available.
- It can give an error upto 1.0 gm/dl.
you can also watch the video of ESTIMATION OF HEMOGLOBIN BY CYANMETHEMOGLOBIN METHOD
.


Comments
Post a Comment